Why Does Honey Have A High Density: Natures Sweet Mystery
Finding Out The Density Of Honey
Keywords searched by users: Why does honey have a high density density of honey water and oil, density of honey and water, is honey denser than oil, relative density of honey, density of spirit, what is the density of honey in kg/m3, honey density g/ml, is honey denser than water
Does Honey Have A Lot Of Density?
Is honey a dense substance? To answer that, let’s delve into the density of honey. Honey is known for its thickness, and its density is typically measured between 1.38 and 1.45 kilograms per liter (kg/L) at a temperature of 20 degrees Celsius (68 degrees Fahrenheit). This range signifies how tightly packed honey molecules are within a given volume, and it provides insight into the substance’s overall heaviness or thickness. Understanding the density of honey can help us appreciate its unique characteristics and applications in various culinary and scientific contexts.
Why Is Honey Denser Than Milk?
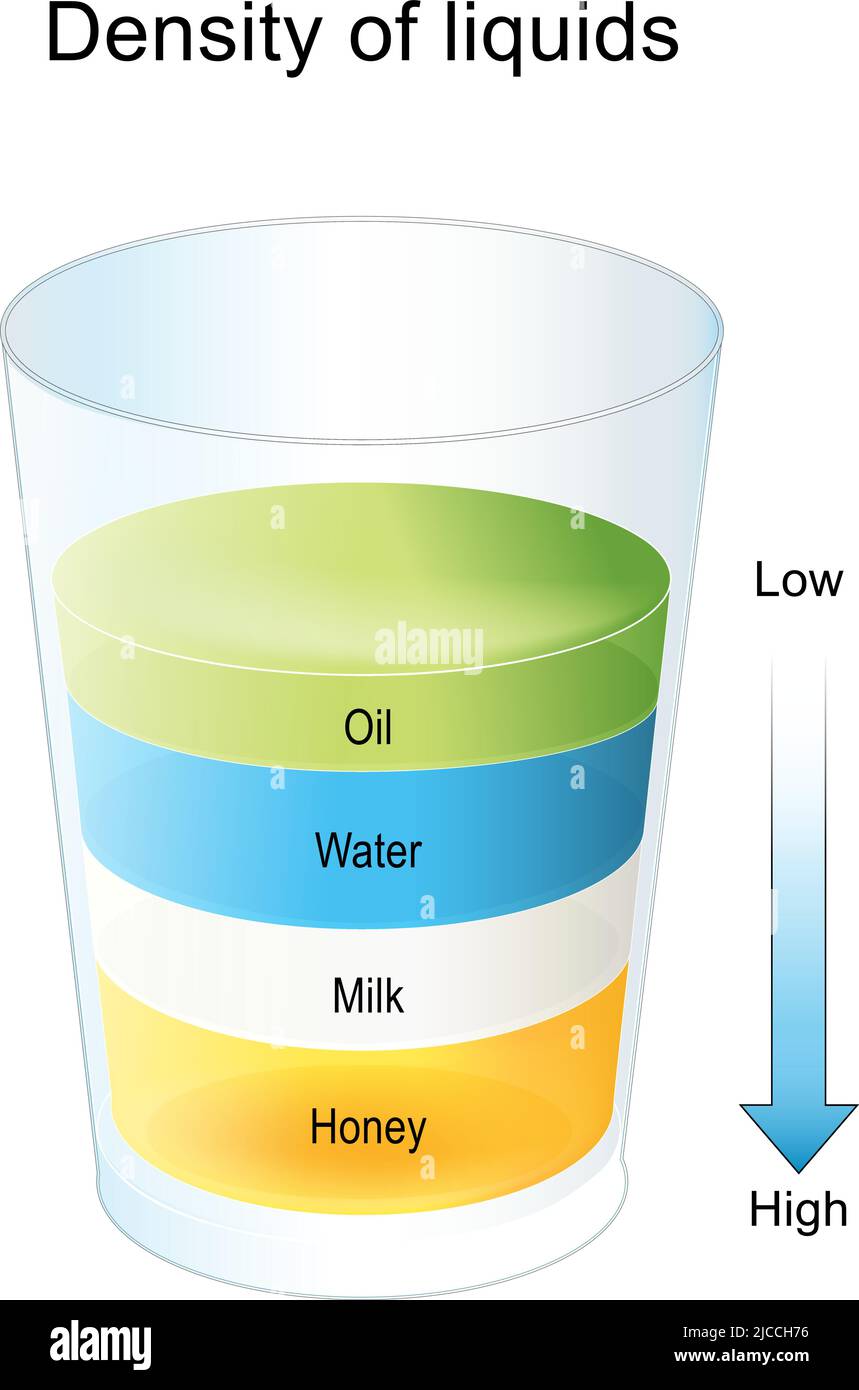
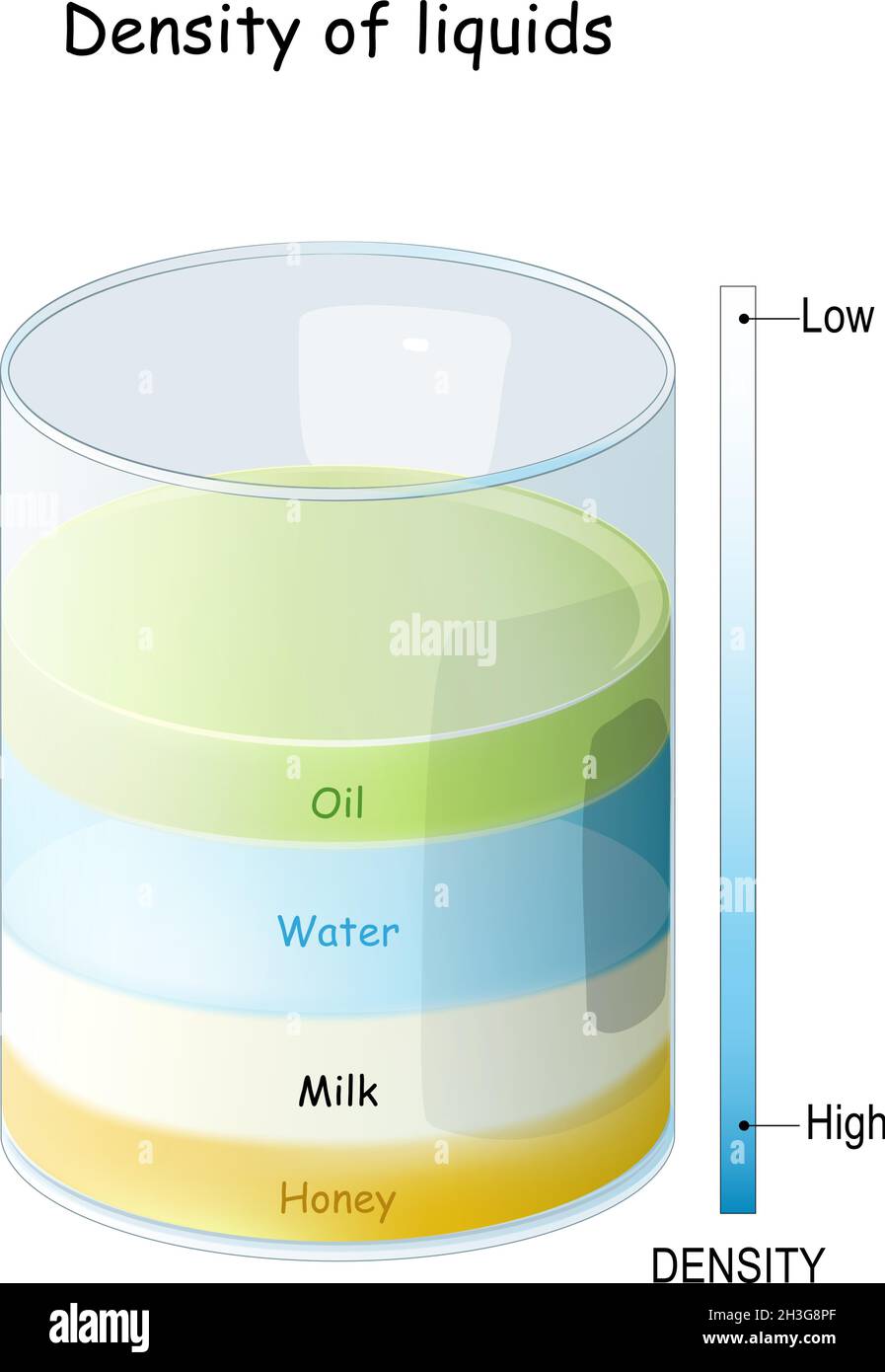
Have you ever wondered why honey is denser than milk? The density of a liquid is often influenced by its sugar content. In this case, honey contains a significantly higher amount of sugar compared to milk. This abundance of sugar molecules makes honey denser than milk. In fact, when you compare honey to other sugary liquids like maple syrup, honey is even denser due to its higher sugar concentration. Milk, while containing some sugar, does not have as much as honey or maple syrup, causing it to sit on top of them when they are layered. To put it in perspective, water is less dense than milk because it contains fewer dissolved substances, and oil is even less dense than water, creating a hierarchy of densities among these common liquids. This phenomenon is due to the varying amounts of dissolved substances in each liquid, which affect their overall density.
Discover 19 Why does honey have a high density



Categories: Discover 89 Why Does Honey Have A High Density
See more here: thichnaunuong.com

The intermolecular force of attraction depends upon the intermolecular distance. Honey is made up of many types of sugars which have very short intermolecular distance and hence there is a larger attractive force between them. Hence its density is greater than water.The density of honey typically ranges between 1.38 and 1.45 kg/L at 20 °C.The higher sugar liquids are the most dense. Honey is more dense maple syrup due to the sugar content, and milk having some sugar, but not a ton, will sit on top of them both. Water is less dense than milk, and oil is less dense than water!
Learn more about the topic Why does honey have a high density.
- Density of honey is greater than the density of water because …
- Honey – Wikipedia
- Simple science at home: Making a density tower! – Local 3 News
- Density Column – Elmer’s Glue
- Honey is more viscous than water. Can you suggest why ? – BYJU’S
- Honey Versus Sugar-Which is Healthier? – Uaex.uada.edu
See more: blog https://thichnaunuong.com/architecture