How Can Iodine Achieve Solubility In Water?
Is Iodine Soluble In Water?
Keywords searched by users: How can iodine be soluble in water is iodine soluble in ethanol, is iodine soluble in hexane, is iodine soluble in oil, is i2 soluble in cyclohexane, is iodine soluble in acetone, is i2 soluble in ccl4, iodine in water reaction, iodine water formula
What Makes Iodine Soluble In Water?
The solubility of iodine in water is a fascinating phenomenon rooted in its interaction with iodide ions. On its own, iodine, being a non-polar molecule, is typically insoluble in water. However, when iodine encounters iodide ions in the vicinity of water-chloroform-hexane interfaces, a remarkable transformation occurs. These two elements react to create triiodide ions. Unlike iodine, triiodide ions are soluble in water but not in the non-polar solvent mixture. This unique chemical reaction at the interface between water and the organic solvents allows us to better grasp the intriguing solubility behavior of iodine in water.
How Is Solubility Of Iodine?
The solubility of iodine in an aqueous cetomacrogol solution exhibits a noteworthy temperature-dependent behavior. Within the temperature range of 20 to 50 degrees Celsius, iodine becomes increasingly soluble as the temperature rises. Interestingly, when this solution undergoes cycles of heating and cooling, the cooled solution retains a greater amount of iodine than what could be achieved through equilibration at a constant temperature. This phenomenon suggests that temperature variations have a significant impact on the solubility of iodine in this particular solution, resulting in an accumulation of iodine in the cooled state beyond what one would expect based solely on equilibrium considerations.
Share 33 How can iodine be soluble in water




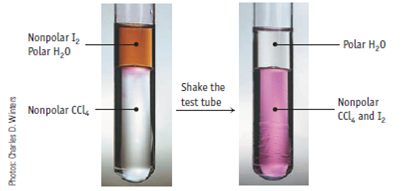

Categories: Collect 50 How Can Iodine Be Soluble In Water
See more here: thichnaunuong.com

Iodine, or I2, is a nonpolar molecule; as such, it is only sparingly soluble in water. However, iodine reacts with iodide ions to form the triiodide ion, which is soluble in water.Although non-polar molecular iodine cannot dissolve in water, it reacts with iodide ion to form something that can: the triiodide ion. When iodide and iodine meet at water/chloroform-hexane surfaces, the triiodide ion formed dissolves in the water, not the non-polar solvent mixture.Over the temperature range 20–50° the solubility of iodine in aqueous cetomacrogol solution increases with rise of temperature. When a solution is heated and cooled the amount of iodine in the cooled solution is greater than can be obtained by equilibration at that temperature alone.
Learn more about the topic How can iodine be soluble in water.
- Is iodine soluble in water? – Quora
- LIKE DISSOLVES LIKE – Purdue Chemistry
- THE SOLUBILITY OF IODINE IN AQUEOUS SOLUTIONS OF NON …
- Why is iodine not soluble in water? – Quora
- Which of the gas is highly soluble in water? – Toppr
- Iodine in drinking-water – World Health Organization (WHO)
See more: blog https://thichnaunuong.com/architecture