How Is Chromic Acid Formed: Potassium Dichromate And Sulfuric Acid Reaction
The Colour Change Of Acidified Potassium Dichromate – The Real Chemist
Keywords searched by users: How is chromic acid formed by the reaction of potassium dichromate and sulfuric acid potassium dichromate sulfuric acid, potassium dichromate + sulphuric acid equation, potassium dichromate and sulfuric acid reaction, potassium dichromate sulfuric acid cleaning solution, why is sulphuric acid added to potassium dichromate, potassium dichromate sulphuric acid colour change, potassium dichromate sulphuric acid ethanol equation, what is chromic acid
How Is Chromic Acid Formed From Potassium Dichromate And Sulfuric Acid?
To create chromic acid from potassium dichromate and sulfuric acid, a specific procedure is followed. First, sodium or potassium dichromate is transformed into a paste by incorporating a small amount of water. Subsequently, sulfuric acid is carefully added to this paste in a gradual, dropwise manner while ensuring continuous mixing. This meticulous process results in the formation of chromic acid. This chemical transformation is essential for various applications and experiments, providing a versatile and valuable substance in chemistry.
How Is Chromic Acid Formed From Potassium Dichromate?
The formation of chromic acid from potassium dichromate involves a specific chemical process. First, either sodium dichromate or potassium dichromate is used as the initial substance. To initiate the reaction, a small amount of water is added to the chosen dichromate compound, creating a paste-like mixture. The critical step in this process is the addition of sulfuric acid to the paste while vigorously stirring. This combination of sulfuric acid with the dichromate compound triggers a chemical transformation that results in the formation of chromic acid. This process took place on February 14th, 2022.
What Happens When K2Cr2O7 Reacts With H2So4?
When potassium dichromate (K2Cr2O7) undergoes a chemical reaction with sulfuric acid (H2SO4), a balanced chemical equation illustrates the transformation:
K2Cr2O7 + 4H2SO4 → Cr2(SO4)3 + 7H2O + 3SO2 + K2SO4
In this reaction, potassium dichromate reacts with sulfuric acid to produce chromium(III) sulfate, water, sulfur dioxide, and potassium sulfate as products. This reaction is commonly employed in various chemical applications and analytical chemistry procedures.
Update 17 How is chromic acid formed by the reaction of potassium dichromate and sulfuric acid



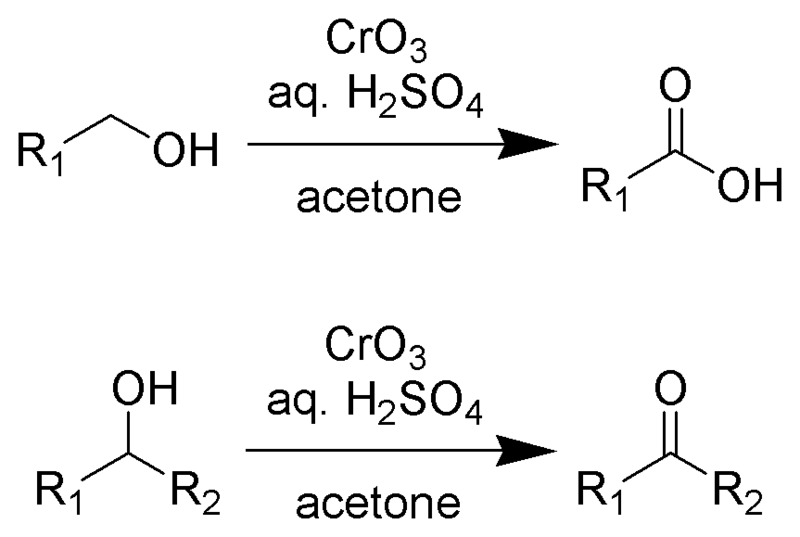
Categories: Details 56 How Is Chromic Acid Formed By The Reaction Of Potassium Dichromate And Sulfuric Acid
See more here: thichnaunuong.com

Learn more about the topic How is chromic acid formed by the reaction of potassium dichromate and sulfuric acid.
- Oxidation by Chromic Acid
- Chromic Acid – Properties, Formula, Structure & Uses of H2CrO4 – BYJU’S
- Liquid Chromic Acid Formula & Examples – Video & Lesson …
- How do you balance this reaction? K2Cr2O7(aq) + H2SO4(aq … – Toppr
- Chromic Acid – an overview | ScienceDirect Topics
- US5096548A – Process for the preparation of chromic acid
See more: blog https://thichnaunuong.com/architecture